Technique Used to Separate Liquids From One Another
This is usually accomplished with a perforated barrier wire screen non-woven fiber or granular media that. Similar to simple distillation fractional distillation is best for separating a solution of two miscible liquids.

Separating Mixtures Overview Common Methods Expii
What is the separating funnel technique.

. The funnel is opened and as soon as the denser liquid has been filtered the tap is closed. By using a separating funnel. Liquids can be described in two ways immiscible and miscible.
What methods are used to separate a solid form a liquid. Moves impurities from one layer to another choose. One way to separate a soluble solid from its solution is to make crystals.
We know that different methods are used for the separation of different kinds of mixtures. However washes and extractions have differences Determine whether each statement applies to washes or extractions. This technique is used to separate an insoluble solid from a liquid.
Sublimation is the process in which a substance directly changes from a solid state to a liquid state. Some methods include. In such cases some special techniques are employed.
- Leaves desired compound in its. The process of sublimation is used to separate those substances from a mixture that sublime on heating. All the mixtures containing two or more liquids can be separated by the following two methods.
Washes and extractions are both techniques that use a separatory funnel to separate liquid layers. Chromatography is used to separate mixtures of coloured compounds. The tap of the funnel is opened and the bottom liquid is allowed to run.
Used to separate two solids where one has magnetic properties. By the process of fractional distillation. The two liquids are now separate.
It uses distillation to fractionate. The method chosen depends upon the type of mixture. The mobile phase may be a.
Filtration may be done cold or hot using gravity or applying vacuum using a Buchner or Hirsch funnel or a simple glass funnel. A major disadvantage of cryogenic separation of CO2 is the amount of energy required to provide the refrigeration necessary for the process particularly for dilute gas streams. Chromatography involves the sample being dissolved in a particular solvent called mobile phase.
Various filtering agents are normally used like filtering paper or other materials. Some substances such as ammonium chloride camphor naphthalene and anthracene are sublime substances. Washes and extractions are both techniques that use a separatory funnel to separate liquid layers.
The two liquids are put into the funnel and are left for a short time to settle out and form two layers. The separation technique used for each liquid depends on the properties of the liquids. Take for example the mixture of sand and water.
Leaves impurities in their starting layer choose. Distillation works because it. This difference in the solubility of sugar and sand in water is used to separate them.
Used to separate two liquids that cannot dissolve in one another immiscible liquid separates two liquids by taking advantage of unequal mass. It is used as a separation technique for mixtures which contain sublimable volatile substances and non-sublimable volatile components. Filtration is a separation technique used to separate the components of a mixture containing an undissolved solid in a liquid.
The most common method of separating a liquid from an insoluble solid is the filtration. Distillation crystallization and chromatography. There are too many substances to easily separate each one.
Chromatography is a separation technique used to separate the different components in a liquid mixture. Distilling an ethanol water solution to produce a more highly concentrated ethanol which could be used as a fuel additive or a higher proof alcohol. The exact method used depends on the purpose of the filtration whether it is for the isolation of a solid from a mixture or removal.
However washes and extractions have differences Determine whether each statement applies to washes or extractions Moves desired compound from one layer to another Choose. Distillation of two liquids can be used to separate a solution of two liquids that have different boiling points. The most basic method would be distillation or boiling one of the liquids and collecting the condensation.
Draw a labeled diagram of a distillation set up that can be used to separate mixtures of liquids with different boiling points. There are different ways to separate mixtures for example by filtration crystallisation distillation or chromatography. Separating immiscible liquids is done simply using a separating funnel.
FRACTIONAL DISTILLATION is used to separate the. Chemical compounds are separated by heating them to a temperature at which one or more fractions of the mixture will vaporize. Distillation is used for the separation of components of a mixture containing two miscible liquids that boil without decomposition and have sufficient differences in their boiling points.
The most common and obvious method is filtration. Fractional distillation is a technique used to separate liquids according to their boiling points. It really depends on which two liquids you are try to separate.
Used commercially for streams that already have high CO2 concentrations typically 90 but it is not used for more dilute CO2 streams. The changing of a solid directly into vapours on heating and of vapours into solid on cooling is called sublimation. Some important separating methods are.
Filtration is used here to remove solid particles from the liquid. Distillation can be used to separate solutions of miscible liquids because the liquids have different boiling points. It can be used to obtain a product that.
When measuring the volume of a liquid what is another way of saying 10 cubic centimeters 10cm3. It was introduced by a Russian Scientist Michael Tswett.

1 4 Laboratory Techniques For Separation Of Mixtures Chem 1114 Introduction To Chemistry

Separating Mixtures Different Methods Distillation Evaporation Centrifugation Lesson For Kids Separating Mixtures Science Lessons Lessons For Kids

Candy Chromatography Easy Candy Science For Kids Cool Science Experiments Candy Science Science For Kids
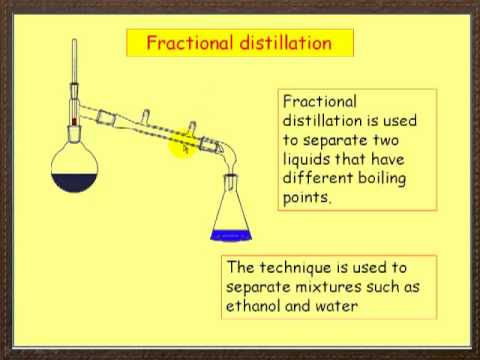
2 10 Separating Mixtures Chemistry Libretexts

Development And Validation Of Liquid Chromatography Rp Hplc Methodology For Estimation Of Efonidipine Hcl Chemistry Help Teaching Chemistry Science Chemistry
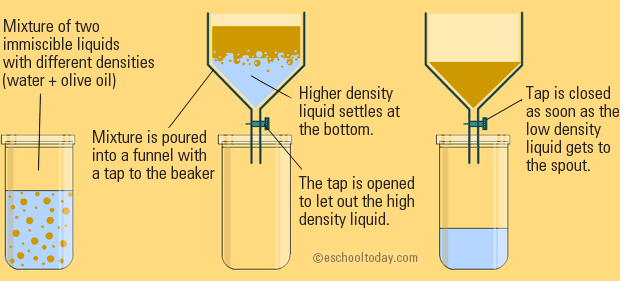
Explain The Mixture Separation Techniques Example
Methods Of Separation Of Mixtures Learn Chemistry Class 9 Amrita Vidyalayam Elearning Network
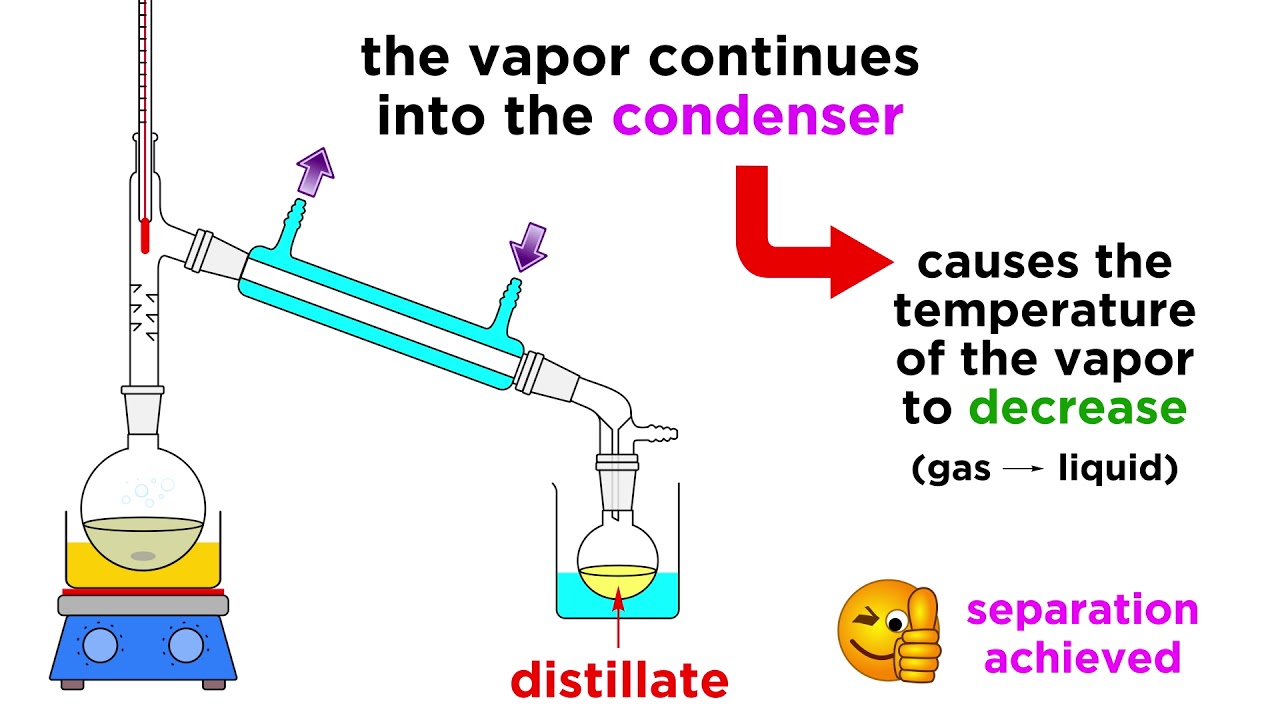
Separating Liquids By Distillation Youtube
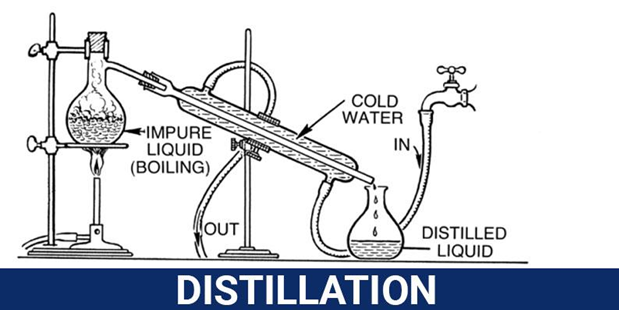
Separating Mixtures Physical Separation Techniques Chemistry

Distillation For Diveling Distillation Chemistry Help Chemistry Experiments

Separation By Fractional Distillation Geeksforgeeks

Separation Of A Mixture Worksheet And Lab Collection Matter Unit Separation Paper Chromatography

Methods For Separating Mixtures Chemistry For Non Majors

Everywhere Where Liquids Have To Be Clarified Or Fine Solids Have To Be Separated From A Liquid Clarifying Disc Stack Centrifuges Separators Centrifuges Stack

1 4 Laboratory Techniques For Separation Of Mixtures Chem 1114 Introduction To Chemistry

Tools To Separate Mixtures 5th Grade Science Matter Science 6th Grade Science

Separating Mixtures Overview Common Methods Expii

Chart Separation Of Mixtures Matter Science Chemistry Lessons Separating Mixtures
Methods Of Separation Of Mixtures Learn Chemistry Class 9 Amrita Vidyalayam Elearning Network
Comments
Post a Comment